2na S 2h2o L 2naoh Aq H2 G
Sodium reacts with water to form sodium hydroxide and hydrogen gas. It has been oxidized.

Which Is The Oxidising Agent In The Following Equation Haso2 Aq Sn 2 Aq H Aq As S Sn 4 Aq H2o I
Single Replacement or substitution Reaction.

. Th 730pm - 920pm. How can we tell that it has reduced if. Up to 256 cash back Get the detailed answer.
H from water has. 2Nas2H₂Ol2NaOHaqH₂g Sodium changes from 0 oxidation state to 1. 2Na s 2H 2 O l 2NaOH aq H 2 g Double Replacement or metathesis Reaction.
View in Time Schedule. For the reaction 2Na s 2H2O l ----- 2NaOH aq H2 g delta H -3686 kJ and delta S -153 JK The maximum amount of work that could be done when 247 moles of. Option C Explanation 2Na s 2H 2 O l 2NaOH aq H 2g Relative molecular mass of NaOH 23 16 1 40smol-1 2 23g of sodium react with water to.
Basic principles of mechanics and experiments in mechanics for physical science and engineering majors. Lecture tutorial and lab components must all be taken to receive credit. Science Chemistry QA Library 2Na s 2H2O 2NaOH aq H2 g Multiple Choice O O O single-displacement double-displacement combination decomposition combustion K.
2Nas 2H2Ol 2NaOHaq H2g Theres nothing like an explosion to help you learn chemistry and the reactivity of alkali metals. CaC2s2H2OlCaOH2aqC2H2g When solid potassium chlorate is heated it decomposes to form solid potassium chloride and oxygen gas. Jointly with G H 101JSIS B 180.
How many grams of sodium will react with water to produce 40 mol of hydrogen in the following reaction. JSIS B 180 AQ G H 101 AQ. 20 PbSO4s 2 H2Ol充电时阳极PbSO4s 2 H2Ol -2e PbO2s 4H aq SO42- aq 阴极PbSO4s 2e Pbs SO42- aq 9氢氧燃料电池P77总反.
It has released and electron. What type of reaction is. A redox reaction.
2Nas 2H2Ol 2NaOHaq H2g In this chemical equation H2O is the oxidizing agent. This problem has been solved. Nas 2H20l 2NaOHaq.
And the outcomes of global health interventions. 2Nas 2H2Ol---- 2NaOHaq H2g 184 grams. The balanced equation which represents the above reaction is.

Use Oxidation States To Identify The Oxidizing Agent And The Reducing Agent In The Following Redox Reaction 2na S 2h2o L 2naoh Aq H2 G Reducing Agent Oxidizing Agent Homework Study Com
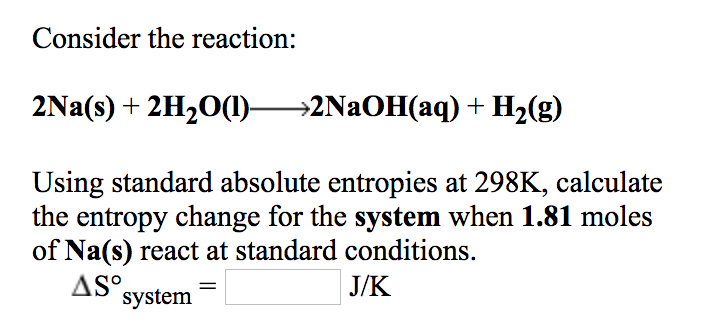
Solved Consider The Reaction 2na S 2h2o 1 2naoh Aq Chegg Com
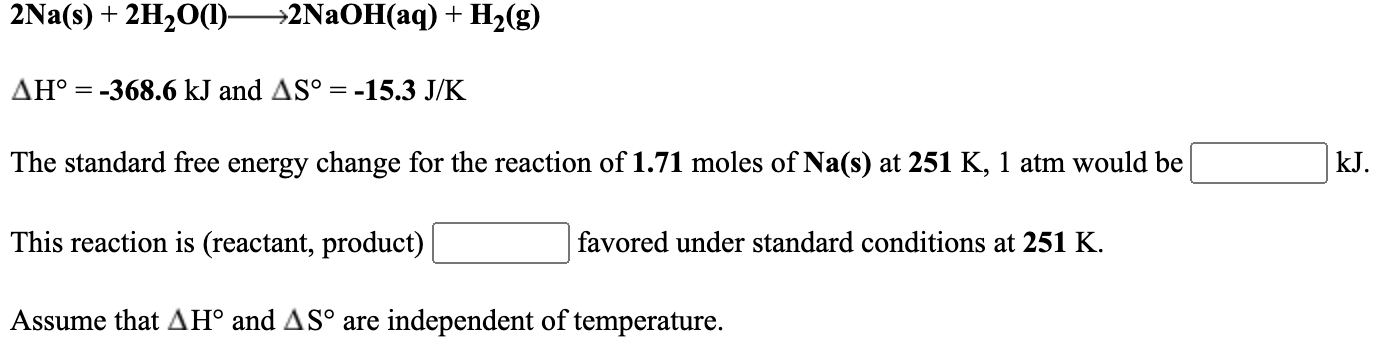
Solved 2na S 2h2o 1 2naoh Aq H2 G Ah 368 6 Kj Chegg Com

Solved Consider The Reaction 2na S 2h2o L 2naoh Aq Chegg Com
Comments
Post a Comment